Oxygen-evolving complex
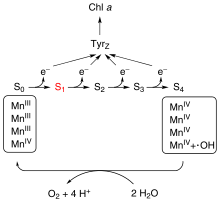
The oxygen-evolving complex (OEC), also known as the water-splitting complex, is a water-oxidizing enzyme involved in the photo-oxidation of water during the light reactions of photosynthesis.[3] OEC is surrounded by 4 core proteins of photosystem II at the membrane-lumen interface. The mechanism for splitting water involves absorption of three photons before the fourth provides sufficient energy for water oxidation.[4] Based on a widely accepted theory from 1970 by Kok, the complex can exist in 5 states, denoted S0 to S4, with S0 the most reduced and S4 the most oxidized. Photons trapped by photosystem II move the system from state S0 to S1 to S2 to S3 and finally to S4. S4 reacts with water producing free oxygen:
- 2 H2O → O2 + 4 H+ + 4 e−
This conversion resets the catalyst to the S0 state.
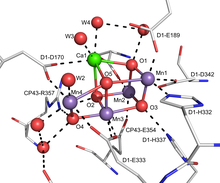
The active site of the OEC consists of a cluster of manganese and calcium with the formula Mn4Ca1OxCl1–2(HCO3)y. This cluster is bound to D1 and CP43 subunits and stabilized by peripheral membrane proteins. Many characteristics of it have been examined by flash photolysis experiments, electron paramagnetic resonance (EPR), and X-ray spectroscopy.[5]
The mechanism of the complex is proposed to involve an Mn-oxide which couples by O-O bond formation to a calcium oxide/hydroxide.[6][7] [8]
References
[edit]- ^ Kok, B.; Forbush, B.; McGloin, M. (1970). "Cooperation of charges in photosynthetic O2 evolution. I. A linear four-step mechanism". Photochem. Photobiol. 11 (6): 467–475. doi:10.1111/j.1751-1097.1970.tb06017.x. PMID 5456273. S2CID 31914925.
- ^ Umena, Yasufumi; Kawakami, Keisuke; Shen, Jian-Ren; Kamiya, Nobuo (May 2011). "Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å" (PDF). Nature. 473 (7345): 55–60. Bibcode:2011Natur.473...55U. doi:10.1038/nature09913. PMID 21499260. S2CID 205224374.
- ^ Raymond, J.; Blankenship, R. (2008). "The origin of the oxygen-evolving complex". Coordination Chemistry Reviews. 252 (3–4): 377–383. doi:10.1016/j.ccr.2007.08.026.
- ^ Johnson, James. "The Origin of Life - The Rise of the Oxygen Evolving Complex". www.chm.bris.ac.uk. Florida University. Retrieved 2020-04-30.
- ^ Abstract : Manganese: The Oxygen-Evolving Complex & Models1 : Encyclopedia of Inorganic Chemistry : Wiley InterScience
- ^ Askerka, Mikhail; Brudvig, Gary W.; Batista, Victor S. (2017). "The O2-Evolving Complex of Photosystem II: Recent Insights from Quantum Mechanics/Molecular Mechanics (QM/MM), Extended X-ray Absorption Fine Structure (EXAFS), and Femtosecond X-ray Crystallography Data". Accounts of Chemical Research. 50 (1): 41–48. doi:10.1021/acs.accounts.6b00405. PMID 28001034.
- ^ Yano, Junko; Kern, Jan; Yachandra, Vittal K.; Nilsson, Håkan; Koroidov, Sergey; Messinger, Johannes (2015). "Light-Dependent Production of Dioxygen in Photosynthesis". In Peter M.H. Kroneck and Martha E. Sosa Torres (ed.). Sustaining Life on Planet Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases. Metal Ions in Life Sciences. Vol. 15. Springer. pp. 13–43. doi:10.1007/978-3-319-12415-5_2. ISBN 978-3-319-12414-8. PMC 4688042. PMID 25707465.
- ^ Oyala, Paul H.; Stich, Troy A.; Debus, Richard J.; Britt, R. David (2015). "Ammonia Binds to the Dangler Manganese of the Photosystem II Oxygen-Evolving Complex". Journal of the American Chemical Society. 137 (27): 8829–8837. doi:10.1021/jacs.5b04768. PMID 26083545.
